- Molecules of organic halogen compounds are generally polar. Due to greater polarity as well as higher molecular mass as compared to the parent hydrocarbon, the intermolecular forces of attraction (dipole-dipole and van der Waals) are stronger in the halogen derivatives. That is why the boiling points of chlorides, bromides and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass.
- For the same alkyl group, the boiling points of alkyl halides decrease in the order: RI > RBr > RCl > RF. This is because with the increase in size and mass of halogen atom, the magnitude of van der Waal forces increases.
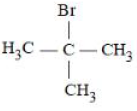
- The boiling points of isomeric haloalkanes decrease with increase in branching.
A) CH3CH2CH2CH2Br (Bromobutane): Molecular mass: 137 u
D) 2-bromo-2-methylpropane: Molecular mass = 137 u, less boiling point than A due to branching.
B) CH3CH2CH2Br (Bromopropane): Molecular mass = 123 u
C) CH3CH2Br (Bromoethane): Molecular mass = 109 u |