The correct answer is option 4. A > D > E > B > C.
The order of basicity of the given nitrogen-containing compounds is:
A. Piperidine > B. Aniline > D. Cyclohexanamine > C. Pyridine > E. Pyrrole
This order is based on the ease of availability of the lone pair of electrons on the nitrogen atom. Piperidine and aniline have their lone pairs on sp3 hybrid orbitals, which are more accessible for donation than the lone pairs on sp2 hybrid orbitals of pyridine and pyrrole. Cyclohexanamine is slightly less basic than aniline due to the inductive effect of the alkyl groups.
Let us look at each of the given compounds
- Piperidine: The lone pair of electrons on the nitrogen atom in piperidine is in an sp3 hybrid orbital, which is large and diffuse. This makes the electron pair less strongly attracted to the nucleus and more easily available for donation.
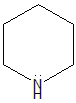
- Cyclohexanamine: The lone pair of electrons on the nitrogen atom in cyclohexanamine is also in an sp3 hybrid orbital, but it is even less accessible than the lone pair on the nitrogen atom in aniline. This is because cyclohexanamine is a secondary amine, which means that it has two alkyl groups attached to the nitrogen atom. The alkyl groups are also electron-withdrawing groups, which further reduce the availability of the lone pair on the nitrogen atom.
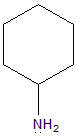
- Aniline: The lone pair of electrons on the nitrogen atom in aniline is also in an sp3 hybrid orbital, but it is slightly less accessible due to the inductive effect of the phenyl group. The phenyl group is an electron-withdrawing group, which means that it pulls electrons away from the nitrogen atom. This makes the electron pair on the nitrogen atom slightly less available for donation.

- Pyridine: The lone pair of electrons on the nitrogen atom in pyridine is in an sp2 hybrid orbital, which is smaller and more localized than an sp3 hybrid orbital. This makes the electron pair more strongly attracted to the nucleus and less available for donation.

- Pyrrole: The lone pair of electrons on the nitrogen atom in pyrrole is also in an sp2 hybrid orbital, and it is even less accessible than the lone pair on the nitrogen atom in pyridine. This is because the lone pair of electrons in pyrrole is involved in the aromatic pi system of the ring. This conjugation makes the electron pair more stable and less available for donation.
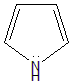
Therefore, the order of basicity of the given nitrogen-containing compounds is:
Piperidine > Cyclohexanamine > Aniline > Pyridine > Pyrrole i.e., |