For a zero-order reaction, the plot of concentration vs time is linear with: |
+ve slope and zero intercept –ve slope and zero intercept +ve slope and non-zero intercept –ve slope and non-zero intercept |
–ve slope and non-zero intercept |
The correct answer is option 4. –ve slope and non-zero intercept. In a zero-order reaction, the rate of the reaction is independent of the concentration of the reactant(s). The plot of concentration versus time for a zero-order reaction is indeed linear, but with a negative slope and a non-zero intercept. Let's consider the general form of a zero-order reaction: A → Products In this reaction, the rate equation is given by: rate = k where "k" represents the rate constant. Since the rate is constant and independent of the reactant concentration, the concentration of the reactant "A" decreases linearly with time. The plot of concentration versus time will have a negative slope because the concentration decreases over time. The non-zero intercept indicates that the reaction does not start from zero concentration; there is a certain initial concentration of reactant present at time zero. This linear plot with a negative slope and non-zero intercept is a characteristic feature of zero-order reactions. 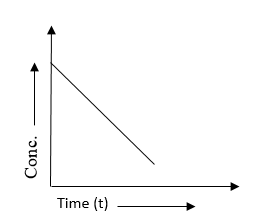 |