The correct answer is option 1. Iodoform test
The Iodoform test is a chemical test used to distinguish between different types of ketones. Here's how it works for these two ketones:
1. Pent-2-one (2-pentanone): When pent-2-one reacts with iodine \((I_2)\) in the presence of a strong base (e.g., sodium hydroxide, \(NaOH\)), it undergoes the iodoform reaction. The result is the formation of a yellow precipitate of iodoform \((CHI_3)\). The reaction can be summarized as follows:
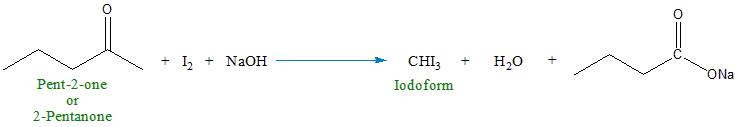
2. Pent-3-one (3-pentanone): Pent-3-one does not undergo the iodoform reaction because it lacks a methyl group attached to the carbonyl carbon (the carbon double-bonded to the oxygen atom). The iodoform test relies on the presence of this methyl group.
In contrast, options 2 (Neutral \(FeCl_3\) test), 3 (Blue litmus test), and 4 (Lucas reagent test) are not specific tests for distinguishing between these two ketones and would not yield distinct results for pent-2-one and pent-3-one. |