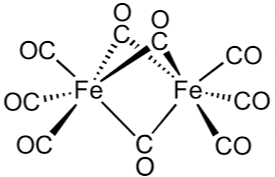
What is the Effective atomic number of Fe in Fe2(CO)9? |
35 36 37 Cannot be calculated |
35 |
Effective Atomic Number = Atomic number of central atoms − (oxidation no. of central atom) + No. of electron taken from Ligands. Atomic No. of Fe = 26 and ′CO′ is a neutral ligand. So, oxidation no. of central atom = 0.
The 3 terminal bonds for each ′Fe′ share 2 electrons each while the middle common bonds share 1 electron.
Total shared electrons = 3×2 + 3 = 9
Now, EAN = 26−0+9 = 35
 |