The correct answer is option 3. A-p, B-r, C-s, D-q
Crystal Field Stabilization Energy refers to the energy difference between the higher-energy set of d-orbitals \((e_g)\) and the lower-energy set of d-orbitals \((t_{2g})\) in an octahedral coordination complex. CFSE is a key concept in Crystal Field Theory and is used to explain the stability and color of transition metal complexes.
In an octahedral complex, the d-orbitals split as follows:
1. Three d-orbitals \((d_{x^2 - y^2}, d_{z^2})\) are raised in energy, forming the "\((e_g)\)" set.
2. The remaining two d-orbitals \((d_{xy}, d_{xz}, d_{yz})\)are lowered in energy, forming the "\((t_{2g})\)" set.
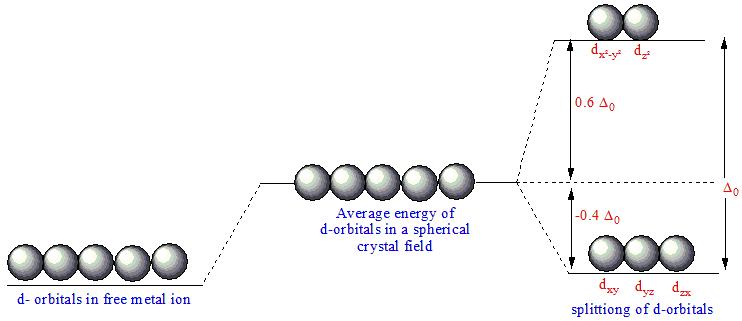
The Crystal Field Stabilization Energy (CFSE) for an octahedral complex can be calculated using the following formula:
\(\text{CFSE = number of electrons in } t_{2g} × \text{ -0.4}\Delta _0 \text{ + number of electrons in }e_g × \text{ 0.6 }\Delta _0)\)
The crystal field splitting parameter, Δo, is determined experimentally and can vary depending on the metal ion and the nature of the ligands surrounding it. The magnitude of Δo determines whether the complex has a low-spin or high-spin electronic configuration. If Δo is large enough to overcome the pairing energy, the complex will adopt a low-spin configuration, with electrons filling the t2g orbitals before pairing up in the eg orbitals. If Δo is small, the complex will adopt a high-spin configuration, with electrons populating both the t2g and eg orbitals before pairing up.
The CFSE has important implications for the stability and reactivity of transition metal complexes. It is a major factor influencing the thermodynamics of complex formation, and it also affects the absorption and emission spectra, magnetic properties, and catalytic activities of the complexes. Additionally, CFSE plays a significant role in explaining the color of transition metal complexes, as the energy difference between the eg and t2g sets corresponds to specific wavelengths of light that are absorbed or transmitted, giving rise to characteristic colors.
In the case of a strong field, low spin complexes are formed and there is a pairing of electrons in the t2g level first then in the eg level. The CFSE for low-spin complexes is given below:
|
Total d-electrons
|
Configuration
|
CFSE
|
|
d0
|
\(t_{2g}^0e_g^0\)
|
\(\text{CFSE = } 0 × -0.4 \Delta _0 + 0 × 0.6 \Delta _0) = 0\Delta _0 \)
|
|
d1
|
\(t_{2g}^1e_g^0\)
|
\(\text{CFSE = } 1 × -0.4 \Delta _0 + 0 × 0.6 \Delta _0) = -0.4 \Delta _0 \)
|
|
d2
|
\(t_{2g}^2e_g^0\)
|
\(\text{CFSE = } 2 × -0.4 \Delta _0 + 0 × 0.6 \Delta _0) = -0.8 \Delta _0 \)
|
|
d3
|
\(t_{2g}^3e_g^0\)
|
\(\text{CFSE = } 3 × -0.4 \Delta _0 + 0 × 0.6 \Delta _0) = -01.2 \Delta _0 \)
|
|
d4
|
\(t_{2g}^4e_g^0\)
|
\(\text{CFSE = } 4 × -0.4 \Delta _0 + 0 × 0.6 ×\Delta _0) = -1.6 \Delta _0 \)
|
|
d5
|
\(t_{2g}^5e_g^0\)
|
\(\text{CFSE = } 5 × -0.4 \Delta _0 + 0 × 0.6 \Delta _0) = -2.0 \Delta _0 \)
|
|
d6
|
\(t_{2g}^6e_g^0\)
|
\(\text{CFSE = } 6 × -0.4 \Delta _0 + 0 × 0.6 \Delta _0) = -2.4 \Delta _0 \)
|
|
d7
|
\(t_{2g}^6e_g^1\)
|
\(\text{CFSE = } 6 × -0.4 \Delta _0 + 1 × 0.6 \Delta _0) = -1.8 \Delta _0 \)
|
|
d8
|
\(t_{2g}^6e_g^2\)
|
\(\text{CFSE = } 6 × -0.4 \Delta _0 + 2 × 0.6 \Delta _0) = -1.2 \Delta _0 \)
|
|
d9
|
\(t_{2g}^6e_g^3\)
|
\(\text{CFSE = } 6 × -0.4 \Delta _0 + 3 × 0.6 \Delta _0) = -0.6 \Delta _0 \)
|
|
d10
|
\(t_{2g}^6e_g^4\)
|
\(\text{CFSE = } 6 × -0.4 \Delta _0 + 4 × 0.6 \Delta _0) = 0 \Delta _0 \)
|
|