- The valence orbitals that is orbitals, are deep buried so, they are not affected by the ligand so, all lanthanides show a few oxidation states, basicity, sharp electronic spectra, etc. so, the statement that the chemistry of different lanthanides is very similar, is true.
- 4f orbital is closer to the nucleus than the orbitals so, electrons in 4f4f orbital feel more attraction so, 4f4f orbital has more density of electrons than orbitals so, 5f5f orbital has high shielding power than 4f4f orbitals. So, the statement that 4f and 5f orbitals are equally shielded, is incorrect.
- d-block elements are known as inner transition elements because they lie in between metals and non-metals so, their shows irregular properties also the valence orbitals that are d-orbitals, are of not deeply buried so, they get affected by ligand So, they show variation in properties such as magnetic and electronic spectra, oxidation states etc.so, the statement that d-block elements show irregular and erratic chemical properties among themselves, is true.
The electronic configuration of La and Lu is as follows:
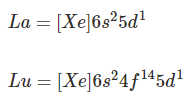 - All lanthanides have lower energy valence shells whereas the actinides have higher energy valence shells, so all lanthanides show similar physical and chemical properties whereas all actinides show variation in physical and chemical properties.
|